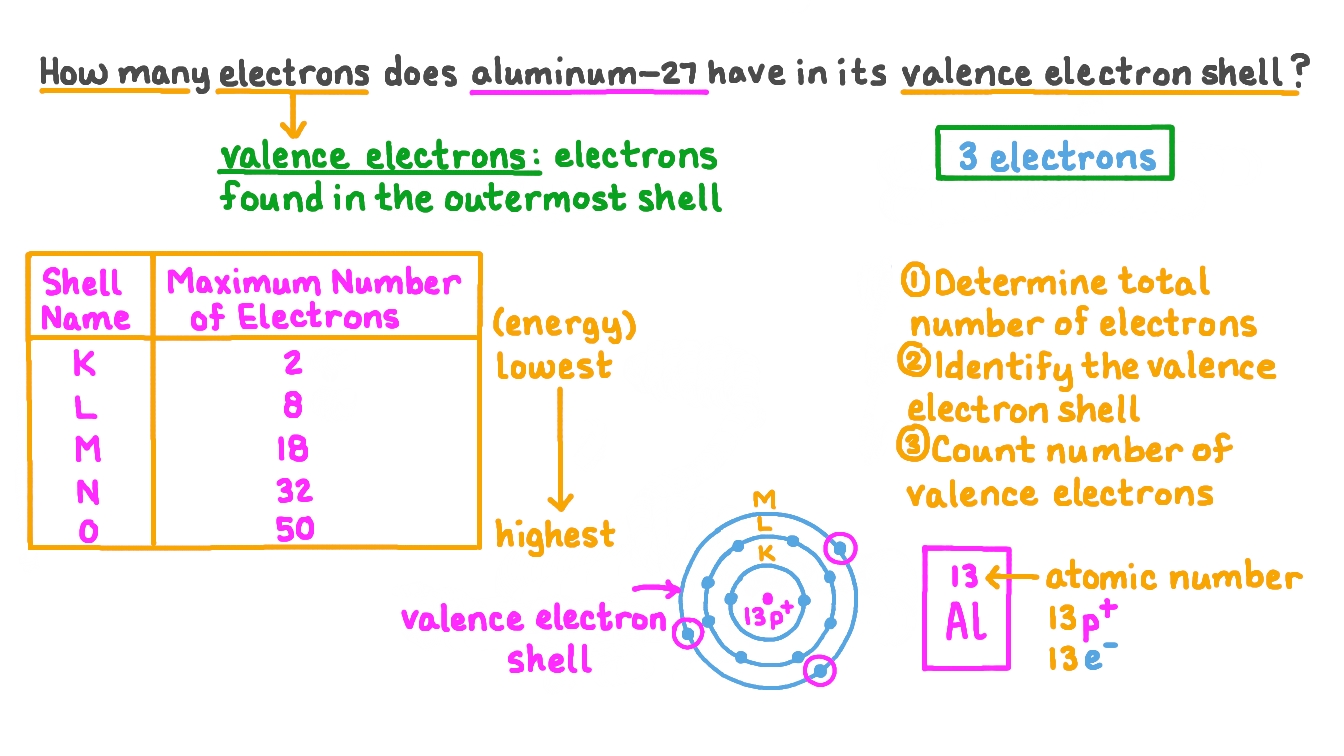
Question Video Determining the Number of Electrons in the Valence
Step 1) Figure out how many electrons the molecule must have. Carbon has 4 valence electrons. Each oxygen has 6 valence electrons. The -2 charge means that there are 2 extra electrons. Total: 4 + (3 × 6) + 2 = 24 electrons. The final answer MUST have this number of electrons‼!
5+ orbital diagram for neon RoomilaDeejay
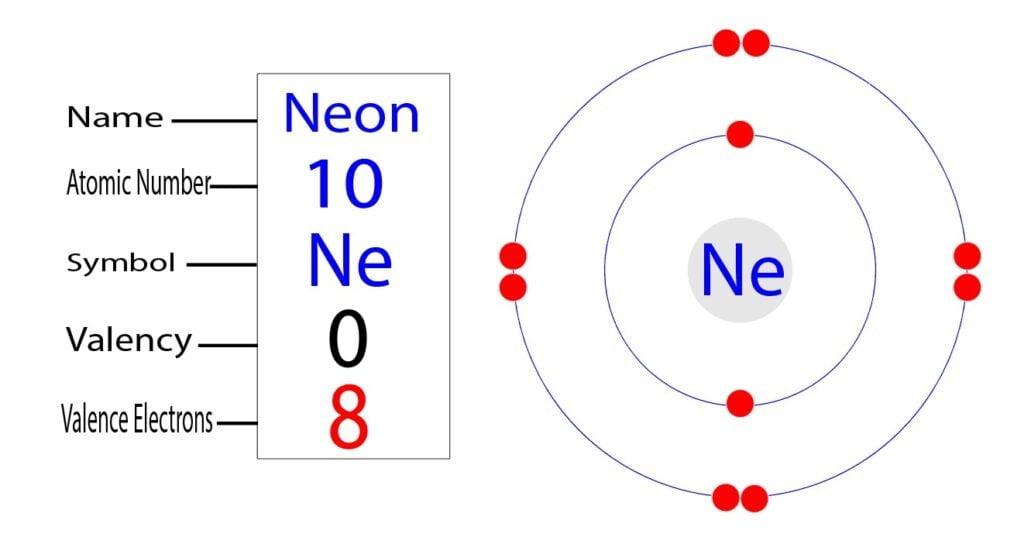
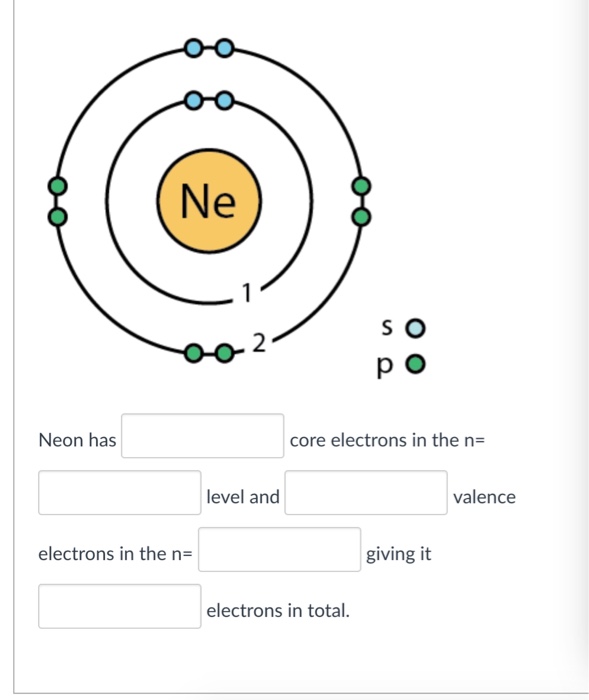
Therefore, neon has 8 valence electrons. Answer b Calcium has electrons in the first, second, third, and fourth energy levels, as indicated by the leading red 1, 2 's, 3 's, and 4, respectively. Valence electrons are those found in the highest occupied energy level.
How many valence electrons does neon(Ne) have?
Please enable JavaScript to access the full features of the site. Glossary Allotropes Some elements exist in several different structural forms, called allotropes. Each allotrope has different physical properties. For more information on the Visual Elements image see the Uses and properties section below. Move to Fluorine Move to Sodium > Neon
How many valence electrons does sulfur(S) have?
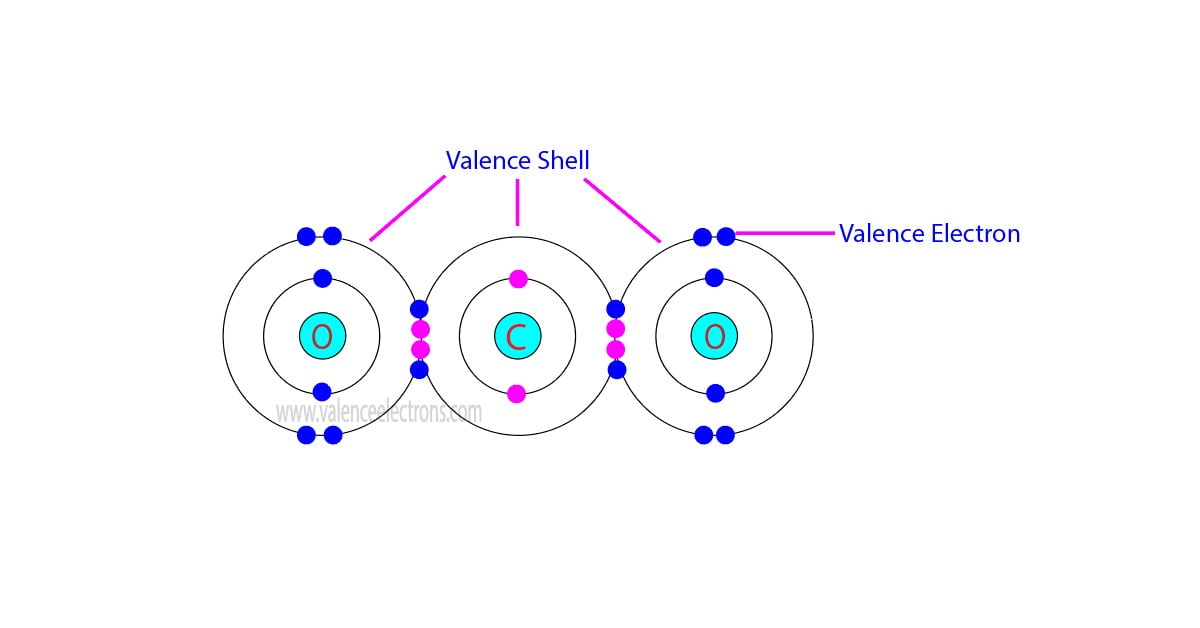
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron.
Quia Electrons and the Periodic Table
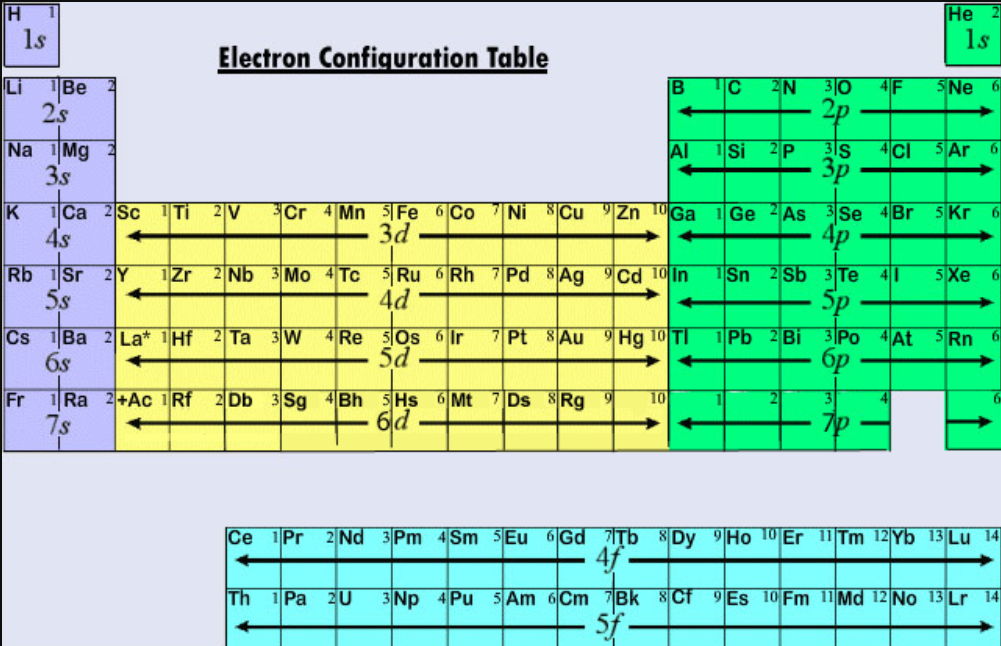
The arrangement of electrons in neon in specific rules in different orbits and orbitals is called the electron configuration of neon. The electron configuration of neon is [ He] 2s 2 2p 6, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)
How Many Valence Electrons Does Helium (He) Have? [Valency of He]
The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.
Valency of Selenium Archives Dynamic Periodic Table of Elements and
There are four simple steps to find out the valence electrons for neon atom which are: Step 1: Find the Atomic Number To find out the atomic number of neon, we can use the periodic table. With the help of the periodic table, we can easily see that the atomic number of neon is 10.
Solved Ne 2 p o Neon has core electrons in the n level and
About Transcript Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions Tips & Thanks
PPT Unit 3 PowerPoint Presentation, free download ID5685070
How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three.. Neon, with its configuration ending in \(2s^2 2p^6\), has eight valence electrons. Valence electrons for transition elements. Transition elements are a bit trickier.

Neon Protons, Neutrons, Electrons Complete Guide
How many valence electrons does neon have? Flexi Says: Valence electrons are the electrons in the outer energy level of an atom that can participate in interactions with other atoms. Each element has different number of valance electrons. The number of valence electrons in an atom is reflected by its position in the periodic table of the elements.

Neon Protons Neutrons Electrons Electron Configuration
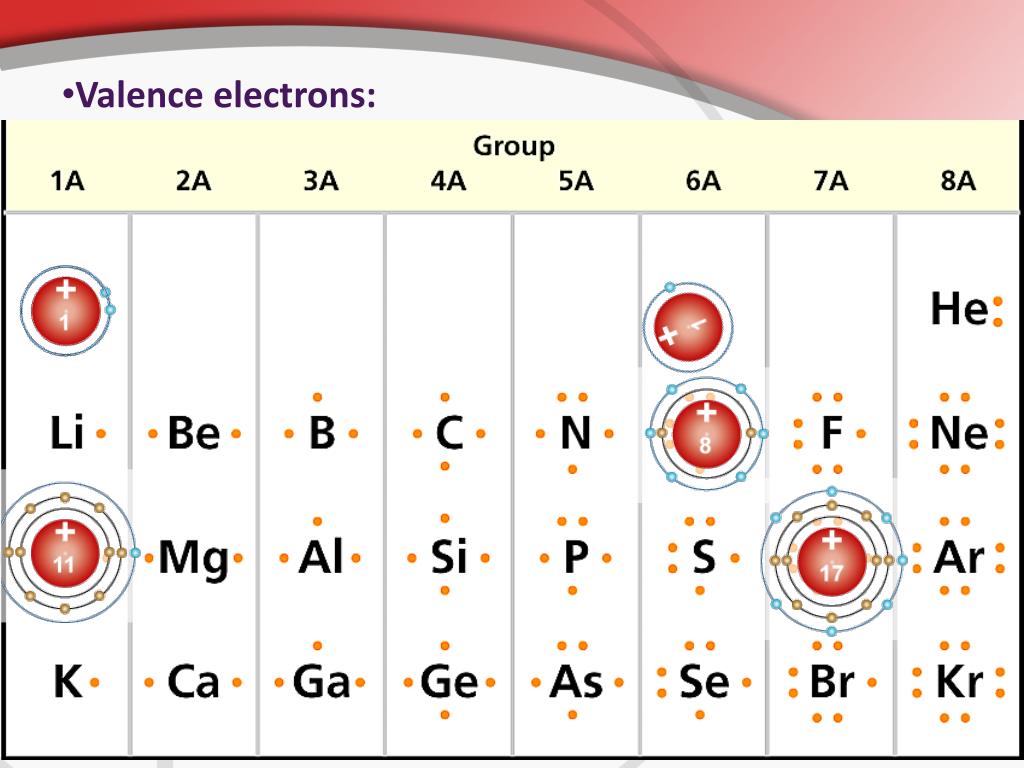
Each element has a number of valence electrons equal to its group number on the Periodic Table. Figure %: The periodicity of valence electrons This table. Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble.

Neon Electron Configuration YouTube
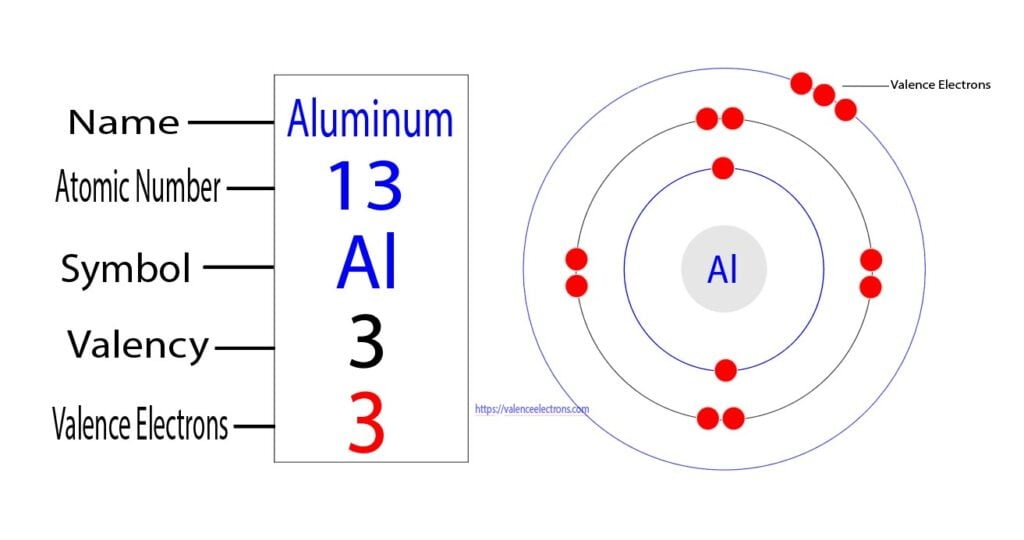
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons.

How Many Valence Electrons Does Neon(Ne) Have?
Get ready to find out how many valence electrons neon has! In this video, we'll explore the fascinating world of chemistry and discover why valence electrons.
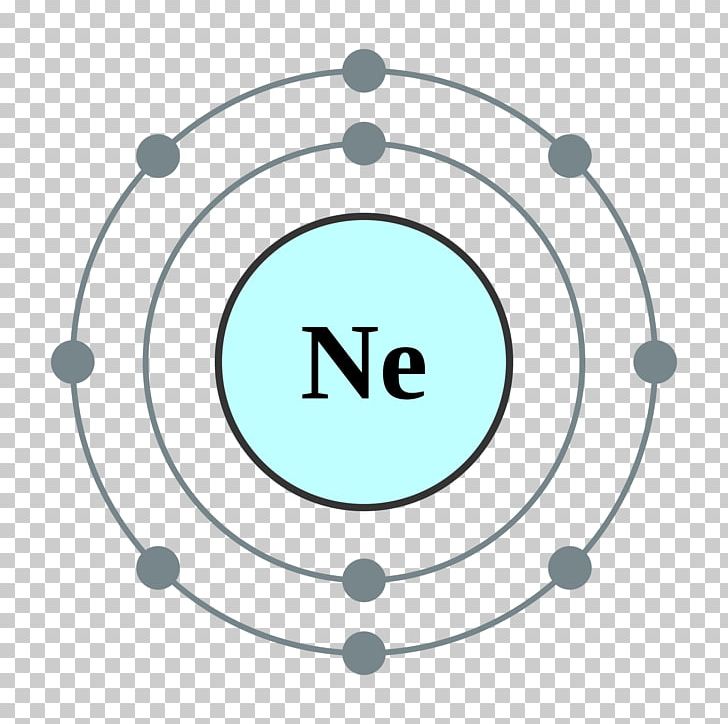
Neon atomic structure Trosadd
Explanation: Neon, Z = 10, has eight valence electrons. This closed shell configuration makes neon supremely difficult to oxidize, and difficult to reduce. The inertness, the lack of reactivity, of this Noble Gas, is a function of its electronic configuration. Answer link To which group of the Periodic Table does neon belong?

How to find the Valence Electrons for Neon (Ne) YouTube
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).
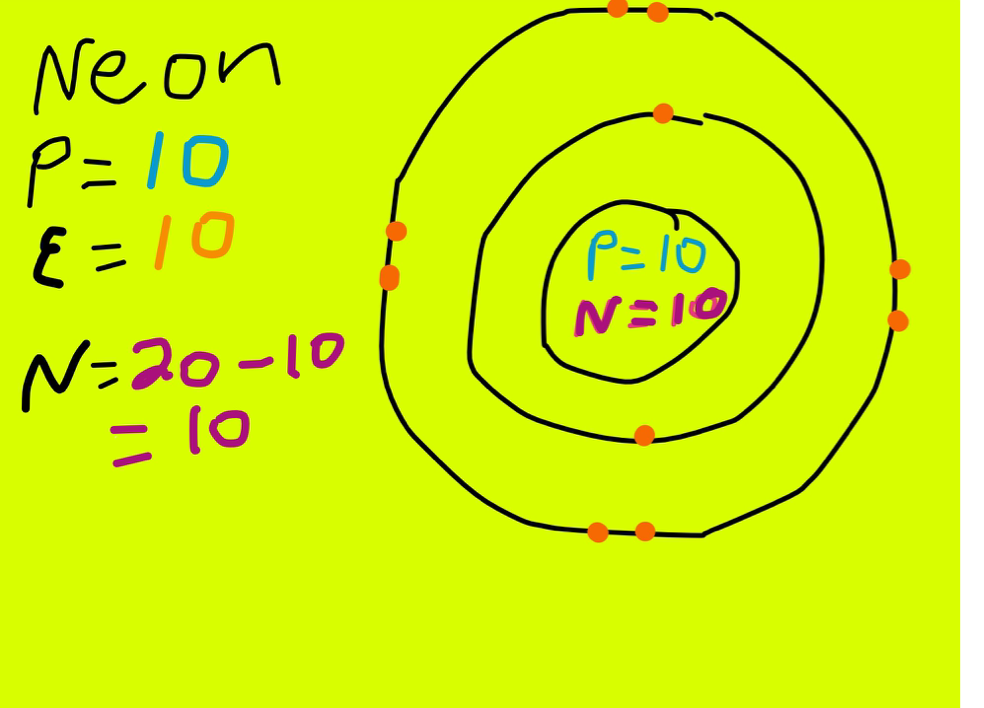
How Many Valence Electrons Are in the Neon Family
There are two ways to find the number of valence electrons in Neon (Ne). The first is to use the Periodic Table to figure out how many electrons Neon has in its valence shell. To do.